

| The Effects of pH and Heat on Vegetable Pigment |
Braden Kay - Lauren Brownlee - Ashley Glover - Olivia Ma
Part I
Part II
Results/Analysis
Conclusion
| PART I |
Materials
Procedure
PURPOSE
The purpose of this experiment is to see how pH levels of certain vegetables change while being cooked in four different mediums (frying, boiling, steaming, roasting), and how the varying pH levels change the coloring of the vegetables. We will analyze how the different method of cooking/heating changes the levels of pH.
BACKGROUND INFORMATION
This experiment is based around the different pigments in vegetables, of which we researched and encountered 3. We were looking at the effects of four different types of heating or cooking methods to see their effects on these pigments, and to see the effects of very acidic solutions and very basic solutions on these same pigments.
Cooking Methods:
Roasting is surrounding food in an oven or other hot inclosure. It relies on the radiation
of heat from the walls and air movement. The air is more stagnant than the other cooking
mediums. The temperature raises to about 500 degrees.
Boiling relies on currents of heat and doesn’t trigger browning reactions. The
temperature of the wateraises to around 212 degrees, and it is the heat of the water that cooks the food.
Steaming is a less dense medium. Vapors in steaming provide more energy, and like boiling, the water used is around 212 degrees and provides steam which is hot enough to cook food in a similar fashion to boiling.
Frying uses a small amount of cooking medium, which is oil. It provides higher heats
than boiling and steaming, though not as high as roasting.
pH pH is the standard measure of proton activity, or measure of acid or base character of a liquid solution. The measure is based on the hydrogen ion concentration of the liquid or solution. The measure is expressed in a number from 0 - 14, and the measure of water is neutral pH 7, the liquids going from pH 0 to pH 6 are acidic, and the liquids going from ph 8 to pH 14 are basic.
Pigments:
Pigment Any coloring matter in plant or animal cells that reflects light of certain wavelengths while also bringing light of other wavelengths without producing appreciable luminessence used to impart color to other material.
Chlorophyll is a green pigment with a ring structure similar to HEME molecule in animal
blood. It is the most common and familiar pigment family. Chlorophyll a is bright
blue-green and is twice as common as chlorophyll b, which is olive. Chlorophyll traps
solar energy and makes it available to form sugar out of water and carbon dioxide in
photosynthesis. Clorophyll is concentrated in discrete cell bodies and is never found in
cytoplasm.
Cartenoids are named for carrots, which they are found in. They are the pigments
responsible for yellow and orange color. They are always found in mixtures, never alone.
Cartenoids play an indirect role in photosynthesis by trapping certain wavelengths of light
and funneling them.
Anthocyanins are the third major class of plant pigments and are a subgroup of phenolic
compounds. Their only functionis to give color to plants. They are water soluable and
stored in cell vacuoles.
HYPOTHESIS
HYPOTHESIS
The methods of cooking which are performed at a higher temperature will change the level of pH more drastically, because the denaturization of the proteins will be increased. Most likely, as the temperature rises, the pH will lower, and the lower the level of pH, or the higher the acidity, the more the pigment will lose its natural color.
MATERIALS
|
a. carrots b. broccoli c. beets a. A frying pan b. grapeseed oil c. A stove top d. a pot for boiling e. a spatula f. a steamer g. a strainer h. a knife i. a cutting board j. a measuring spoon k. multiple bowls a. pH tape b. a camera c. Thermometer d. acetic acid e. baking soda |
PROCEDURE
- Rinse and peel the vegetables
- Cut each vegetable into slices (2 cups of slices 2 cm thick)
- Take a photograph of the raw vegetable for color.
- Blend 1/2 cups of sliced vegetables in the blender on the lowest level of power, along with 1/4 cups of water.
- Put the pureed vegetables into a strainer, and mash it into the netting until there is enough liquid to measure the pH.

- Take the blended vegetables and measure the pH with the pH strips.
- Take the remaining 1 ˝ cups of sliced raw vegetables (that are NOT blended) and cook.

a. Put 2 tablespoons of vegetable oil in an aluminum frying pan and heat until ready
b. Put in 1 ˝ cups of sliced raw vegetables into the pan
c. Cook and stir for 5 minutes.
d. Remove the sliced vegetables from the frying pan - Take a photograph of the cooked sliced vegetables for color comparison
- Blend the 1 ˝ cups of cooked vegetables on low power
- Take the pH of the blended vegetables with the pH strips.

- Repeat steps 1-9 for carrots, broccoli, and beets.
- Repeat steps 1-5
- Take the remaining 1 ˝ cups of sliced vegetables and cook.
a. Bring 6 cups of water to boil in an aluminum pot
b. Place the 1 ˝ cups of sliced vegetables into the boiling water
c. Leave for 8 minutes
d. Remove vegetables from the boiling water - Repeat steps 7 - 10
- Repeat steps 1 - 5
- Take the remaining 1 ˝ cups of sliced vegetables and cook
a. Cover the vegetables with 2 tablespoons of vegetable oil
b. Place on an aluminum sheet in a 500 degree oven
c. Cook for 10 minutes
d. Remove the sheet from the oven - Repeat steps 7-10
- Repeat steps 1-5
- Take the remaining 1 ˝ cups of sliced vegetables and cook
a. Boil 2 cups of water in an aluminum pot.
b. Put a metal strainer on top of the pot.
c. Place the 1 ˝ cups of vegetables in one layer inside the strainer.
d. Cover the pot and steam for 10 minutes.
e. Remove sliced vegetables from steamer.

- Repeat steps 7 - 10
| PART II |
Materials:
Procedure:
PURPOSE
The purpose of Part II is to see how different levels of pH change the color of the vegetables. By adding ingredients to our purred vegetables, which have very extreme, and drastically different levels of pH, we will be able to tell if pH has any effect on vegetable color at all.
HYPOTHESIS
When vegetables are added to solutions these solution with the lower pH will have a greater color change.
MATERIALS
a. beets
b. carrots
c. broccoli
a. pH measuring tape
b. acetic acid
c. baking soda
d. bowls
e. camera
PROCEDURE
- Slice the vegetable into cubes, and put into the blender on low power until completely free of solid pieces.
- Put the vegetable mush into a strainer, as in Part I, and press down with wooden spoon
until enough liquid has accumulated in the bowl beneath it.

- Take a picture of the liquid color of raw vegetables.
- Split the liquid into three parts, in three separate bowls.
- Take the pH of the acetic acid and the baking soda
- In one bowl, add 4 tablespoons of water to serve as the control. In the second bowl, add 4 tablespoons of acetic acid (vinegar) In the third bowl, add baking soda, that is dissolved in lukewarm water. Add 4 tablespoons of this mixture.
- Let the three bowls sit for 10 minutes. After 5 minutes take observations and pictures of the three mixtures. And again after 10 minutes take observations and pictures of the three liquids.
- Take a picture of the liquid color

- Repeat steps 1 - 9 for each vegetable.
RESULTS / ANALYSIS
| Puree | Steaming | Roasting | Frying | Boiling | |
| Broccoli | 5.0 | 5.3 | 4.8 | 5.3 | 4.9 |
| Beets | 5.0 | 6.0 | 5.7 | 4.3 | 5.1 |
| Carrots | 5.1 | 5.6 | 5.4 | 4.9 | 5.6 |
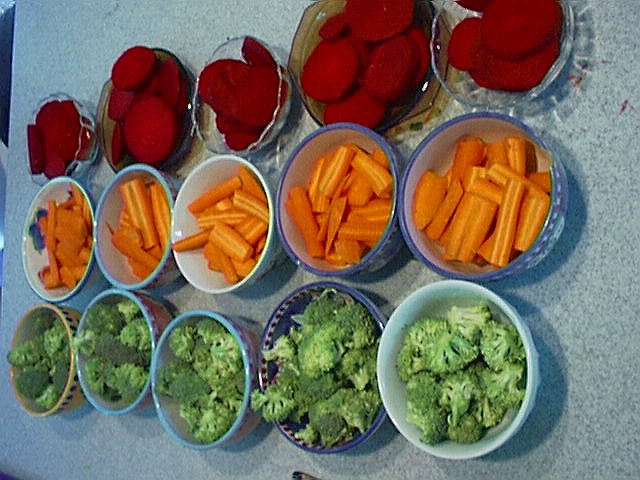
| These three vegetables are the actual vegetables we used in our PART I experiment. The colors of these raw, and peeled carrots, broccoli, and beets are very bright and distinct. |
BROCCOLI

| This is the broccoli that we used for the Part I experiments. The color of this broccoli is what we used to compare all of the cooked vegetables in the experiments. The green color is caused by the chlorophyll pigments. |
The pigments in broccoli which give it its green color are chlorophyll, and these are by far the most abundant pigment. Chloryphyl is easily altered by heat and cooking because of its magnesium atom center. This atom can be "nudged out" fairly easily if heat is applied and can be replaced by hydrogen ions or other metal ions. The heat breaks fown the cell structure in the pigment, and allows an ion, usually hydrogen, to take the place of magnesium which therefore changes the color. This also opens up the structure of the molecule and exposes the plants own acids to the chlorophyl which in turn makes the plants dull green. This is why when a plant is overcooked, it turns that color. But when the broccoli was steamed and boiled, the color was very vibrant after being cooked. Those vegetables that were roasted and fried were browned. The magnesium center and the phytyl group, or hydrocarbon tail which is attached to the molecule and allows chlorophyll to be soluable in lipids, make the broccoli more susceptible to color change.
| This is the broccoli which has been roasted in the oven at 500 degrees. The edges of the vegetables are obviously browned and the entire stalk has turned to a more olive green color. It also seems to have lost some of its moisture and looks more shriveled. | This is the broccoli which has been steamed and it seems like the stalks especially have turned a darker green. The tops are also a little bit darker and a more distinct green | This is the broccoli which has been fried in hot oil and like the roasting, the tops are a little bit discolored, but are more black than brown. | This is the broccoli which has been boiled and it is very obvious that these pieces of broccoli are a lot brighter than the other methods. It is more similar to the steaming than the others, but still has a brighter appearance. |
5 MINUTES.....................10 MINUTES
| These photos are the broccoli juices after 5 minutes. Here, the acetic acid is very obviously lighter and the baking soda is basically the same color as the control. | These are the solutions after 10 minutes. The acetic acid seems to have gotten even lighter than before. The baking soda got a little bit darker, and a little bit olive green. |
These photos show the difference in color when solutions of high acidity and solutions of high basidity are added to the raw broccoli juices. We noticed that the broccoli juice that had acetic acid added to it turned almost immediately to a much lighter shade of green and started to have the sediments clump together in what looked like some kind of curdling reaction. The broccoli juice that had baking soda added to it didn't seem to have any immediate reactions. After 5 minutes, there was a very obvious separation in the bowl of juice, and the color was considerably lighter. In the bowl with baking soda and broccoli juice, the color had gotten only slightly darker than the control, and had very similar properties to the control, such as small pieces of broccoli all settling at the bottom, and a lighter ring of color on the outside of the bowl. After 10 mintes, we saw that the acetic acid bowl was even lighter, and more curdled, and the baking soda bowl had pretty much stayed the same.
BEETS

| This is the color of the raw beets which we compared all the different cooked vegetables to. The very rich deep red color is caused by the betacyanin pigment which is a type of anthocyanin pigment. |
The pigment which colors the beets the strong red color is betacyanin, a type of anthocyanin. Unlike the cartenoids in carrots, betacyanins are very easily and radically altered during most cooking processes. They are known to change color completely, especially with changes in pH. Because anthocyanins are water-soluable, beets are susceptible to color changes in water cooking methods as well as roasting and frying. That is why in boiling, the water is the same color as the beets, the water solubility allows the pigments to come out. We also noticed in the boiling and steaming that the color was incredbily vibrant and bright after being cooked in these manners. We see that the beets cooked by roasting and frying are darker, probably due to the browning effects that take place in those mediums.
| These are the beets which have been roasted and they appear to be much darker than the raw vegetables. Some brown and black areas are visible but mostly the deeper, darker red color is apparent. | These beets have been fried and are an even darker red than the roasted beets. Again, there are some browned spots, but they are generally much darker than the raw beets. | These beets have been boiled and are extremely different than the other cooked beets. They are an extremely bright red and have a much lighter tone than the others. Also, the water in which they were cooked in has turned the same shade. | These beets have been steamed and are bright like the boiled beets but not as light in tone. They are almost as dark as the roasted/fried beets but do not have the browning component. |
5 MINTES.....................10 MINUTES
| Like the broccoli, it is evident after 5 mintues (PHOTO 1) that the acetic acid made the juice lighter. Although, it is not nearly as drastic in its paleness as the broccoli. In the baking soda/juice solution, there is an extremely noticeable change, and after 5 minutes, it is very dark. | After 10 minutes almost everything is the same, and the acetic acid and beet juice solution looks about as light as it did at 5 minutes. The baking soda and beet juice solution seems to have turned even darker than before. This solution is considerably darker than the control. |
In Part II, we did the same procedure to the beets as we did to the broccoli, and got similar results, with a slight variation. Just like the broccoli, when the acetic acid was added to the beet juice, a noticeable color change was noted, but not quite as drasticly light quite as quickly. In the beet juice with baking soda added, there was no real observations made about immediate effects of the baking soda. After 5 minutes, though, we noticed a lighter tone in the beet juice with acetic acid, and a suprisingly darker shade of red in the juice with baking soda. Again, after 10 minutes we observed a very noticeable darkness in the beet juice with the basic solution and a lighter more reddish color in the acidic solution. On researching anthocyanins, we found out that these pigments are known for the drastic color change having to do with pH. Anthocyanins are known for becoming more red when exposed to acid and more dark blue when exposed to bases. This seemed to prove true with our beets.
CARROTS

| These are the carrots we used for the experiments in Part I. The pigments that color these vegetables this deep orange are cartenoids. They are very stable, and all of the cooked carrots are compared to these raw, peeled, vegetables. |
The pigment cartenoid was originally named this because of its prominence in carrots and give it its bright orange color. This type of pigment is fat soluble and often found in special pigment bodies called chromoplasts. The chemical structure of cartenoid is considered to be very stable and therefore is less affected by outside forces and doesn't change color very easily. We found it to be relatively unaffected by cooking and heat, and didn't seem to change color very much. The boiling and steaming methods had the least color change, although they seemed a little bit brighter, while the roasting and frying methods seemed to have more results due to the browning effect discussed later.
| These carrots have been boiled and seem more yellow than the raw carrots. Not only are they lighter, but they seem more yellowish in color than orange. | These carrots have been roasted, and are shriveled and darker on the outside, but on the inside (where we cut) the portions which were originally a light orange now appear white. They are also browned | These carrots have been steamed and are a darker, brighter orange than the rest of the carrots. They appear the most similar in color to the raw carrots. |
5 MINUTES.....................10 MINUTES
| Again, like in the broccoli and the beets, these is an immediate change in the carrot juice that had acetic acid added to it. It becomes lighter, while the juice with baking soda, seems to be a little bit darker. | After 10 minutes, the baking soda and carrot juice appears a little bit darker than before, and darker than the control. The carrot juice with acetic acid is again, lighter. |
In the carrots for Part II, we had results and observations very similar to those in Part II of the broccoli. When first putting in the acetic acid, we noticed a pretty drastic lightening of the color of carrot juice, and not much of a change from the control when the baking soda solution was first added. After 5 minutes, the curdling effect had definetly set in, and all the carrot pulp seemed to be forming clusters while a clear liquid had formed at the top. After 10 minutes, everything was fairly steady, and the acetic acid and carrot juice mixture was again considerably lighter than the control and the baking soda carrot juice mix. The baking soda mix was also a little bit darker than the control, but not very much.
CONCLUSION
During the process of this experiment, we learned many things about the nature of both pH and pigment. Most importantly we realized that contradictory to our original hypothesis for Part I, pH of a vegetable does not change significantly when cooked in any medium; however, heat and pH separately, change the color (pigment) of the vegetables. This means that the manner in which pH and heat change the color of vegetables are very different. If reactions were similar, than the pH of a vegetable would change when the color changes during a heating process. But because, the reaction is not similar heat must have changed the pigment in the vegetables in a manner without using the pH. Another reason the reactions do not correlate is because in Part II, the pH changed the pigments of the vegetables without heat.
Heat seems to change the pigments in a couple of ways. Proving our original hypothesis incorrect, the process of heating does not change the structure of membrane proteins. This would allow the proteins to change shape in order to allow hydrogen ions to pass through and change the pH. This did not happen and the pH did not change because of that. Heat changed the pigment in a variety of ways, one way was by increasing the wild motions and randomness of the protein atoms that circulate. This random motion becomes more energetic than the hydrogen and covalent bonds in the pigments that hold them together can sustain. This results in a breakdown of pigment molecules. In boiling and steaming, the cells expand suddenly, and gases escape from the spaces in between the cells. This change occurs in the first few seconds of cooking, and the air pockets change the color of the chloroplasts when these molecules have been collapsed by the sudden rush of heat on to the vegetable. Vegetables become brighter when steamed or boiled because their molecules become broken down, and are now more exposed and more readily visible. On a molecular level this means that the cooking changes the protein structure in the vegetable from quaternary to tertiary or secondary structure. The structure does not change to primary, because if it did the pH would change due to the breaking of hydrogen bonds, which are present in secondary structure of proteins. The pH would change because the hydrogen gradient in the vegetable proteins would change. The gradient would differ because the hydrogen ions, previously from the hydrogen bonds would move across the protein membrane.
In water related cooking, such as steaming and boiling, the is a less drastic temperature change as the vegetables are exposed to the heat. Because the temperature stays the same, and does not lose heat it takes less time to cook, and the enzymes within the vegetables which must work on the alteration of pigments. These enzyme have less time to work on the pigments which results in and less color change. It was in the boiling and steaming cooking methods that the least change was noticed and when it was, the vegetables were usually brighter, but in the roasting and frying we noticed what are called browning reactions. Browning reactions are the processes of caramelization, also known as the Mallard reaction. In these processes wherein the heated sugar, turns into a different structure through a series of chemical reactions which aren't understood very well. This transformation includes the breaking down of the sugars into hundreds of different reaction products, and often results in a better taste. The reason that these reactions did not occur in the steaming or the boiling is because Maillard reactions can only take place at temperatures usually above 315 degrees, and both boiling and steaming are 212 degrees.
In the process of Part II which consisted of adding solutions of different pHs to observe color changes; a different type of alteration occurred. The introduction of pH increases hydrogen ions, or protons, in the cell, and this results in the denaturalization of proteins, or the change of shape. The new hydrogen ions creates a gradient which is different than before and the proteins will change accordingly. This changed amount of ions makes it more likely that the bonding of proteins will be interrupted and the protein will unfold and lose its structure. We originally believed that heat would make this happen, but it did not. The pH solutions increased the amount of ions and the likelihood of protein denaturalization and a structure change. The denaturalization allowed the hydrogen ions to move around and this changed the shape of the protein and the acidity of the cell, which in turn changed the color of the vegetable juice. We saw that with the broccoli, the chlorophyll got both brighter and duller in response to the change in pH. We saw that with beets, the anthocyanin caused them to get both more light and red, and darker and more purple/blue. It is known that cooking doesn't usually have "trends" like this or cause changes like these using heat.